Thermochemistry
- When a reaction occurs, it may either give out energy, in the form of heat (exothermic), or take in energy (endothermic). The amount of energy that is given out or taken in can be measured as the Enthalpy Change of a reaction (ΔH). A negative ΔH means an exothermic reaction and conversely a positive ΔH means an endothermic reaction. Enthalpy change can be shown in an Enthalpy Level Diagram.

ΔH can be qualitatively measured, to find the enthalpy change in KJ mol-1. For example, the enthalpy change for the reaction
CH4 + 2O2 → CO2 + H2O is-890.3 KJ mol-1 .ΔH varies according to environmental conditions, so, in order to standardise the measurement and recording of enthalpy changes, certain standard conditions are referred to:
- Temperature: 298 °K (25°C)
- Pressure: 1 Atmosphere (roughly 1.01 ×105Nm-2)
- Concentration: 1 mol dm3
Experimental Determination
- The ΔH for a reaction can be determined by experimental means by measuring the temperature change of known volume or mass of a solution caused by the change in energy of a reaction. A Bomb Calorimeter may be used to make this measurement. Using this apparatus is a highly accurate way of determining the enthalpy change of combustion, but the conditions inside it are not standard so the result needs to be modified.
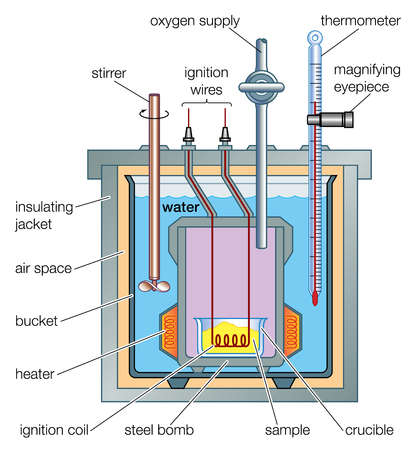
- The following equation can be used to calculate the enthalpy change:
ΔH = M × C × -ΔT
- Where M is the mass or volume of the solution being used to test the energy change, C is the Specific Heat Capacity of the solution, (for example the specific heat capacity of water is 4.1855 J g-1 K-1), and ΔT is the change in temperature. The sign of ΔT is reversed to reflect the fact that ΔH represents the enthalpy change of the test substance not the surroundings.
- There are three different kinds of enthalpy change:
- ΔHf
OStandard Enthalpy Change of Formation: The enthalpy change when 1 mole of a compound in its standard state is formed from its elements in their standard states, under standard conditions. - ΔHc
OStandard Enthalpy Change of Combustion: The enthalpy change when 1 mole of a compound in its standard state is completely combusted in excess oxygen, under standard conditions. - ΔHr
OStandard Enthalpy Change of Reaction: The enthalpy change of a reaction as written in the equation, under standard conditions.
- ΔHf
Hess’s Law
Hess’s Law states that the enthalpy change of a system is independent of the route taken as long as the initial and final conditions are the same.
This means that, for example, the enthalpy change of a reaction can be calculated if given two intermediate reactions that overall result in the same products and reactants.

- In the example above
ΔH = ΔH1 + ΔH2 .